 Luminostics is a medical diagnostics company that specializes in rapid, accurate self-tests for infectious diseases that can be carried out by consumers at home. The company’s expressed mission is to “increase healthcare accessibility and efficiency by designing, developing, manufacturing, and delivering products that enable affordable, widespread access to actionable health testing with immediate follow-up.”
Luminostics is a medical diagnostics company that specializes in rapid, accurate self-tests for infectious diseases that can be carried out by consumers at home. The company’s expressed mission is to “increase healthcare accessibility and efficiency by designing, developing, manufacturing, and delivering products that enable affordable, widespread access to actionable health testing with immediate follow-up.”
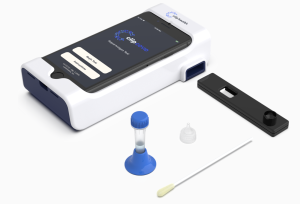 In recent months, Luminostics has become one of the global leaders in innovative
In recent months, Luminostics has become one of the global leaders in innovative
Covid-19 testing methods. Their unique approach involves an iOS/Android app that integrated with an inexpensive adapter, using nanochemistry and signal processing artificial intelligence to detect and measure viruses with a high level of sensitivity. Before the emergence of Covid-19, Luminostics had been experiencing success with accurate testing for HIV, herpes, chlamydia, and other STDs.

In April of 2020, Luminostics partnered with another company called Sanofi to collaborate on a self-testing solution for Covid-19. Sanofi specializes in clinical research and strong testing capabilities. The goal was to create an app-based testing method that could run on any smartphone, eliminating the need for healthcare professionals to get involved in the testing process for the average consumer/patient.
Learn More:
